Rapid-C: Real-time viable particle counter and your solution to the revised Annex 1
The revised European Union GMP Annex 1: Manufacture of Sterile Medicinal Products is effective from 25 August 2023 and still poses significant challenges to pharmaceutical manufacturers, emphasizing the need for advanced technologies to enhance contamination control strategies (CCSs). The revised guidelines underscore the importance of robotics, automation, and the adoption of rapid microbiological methods to elevate the efficiency of CCSs.
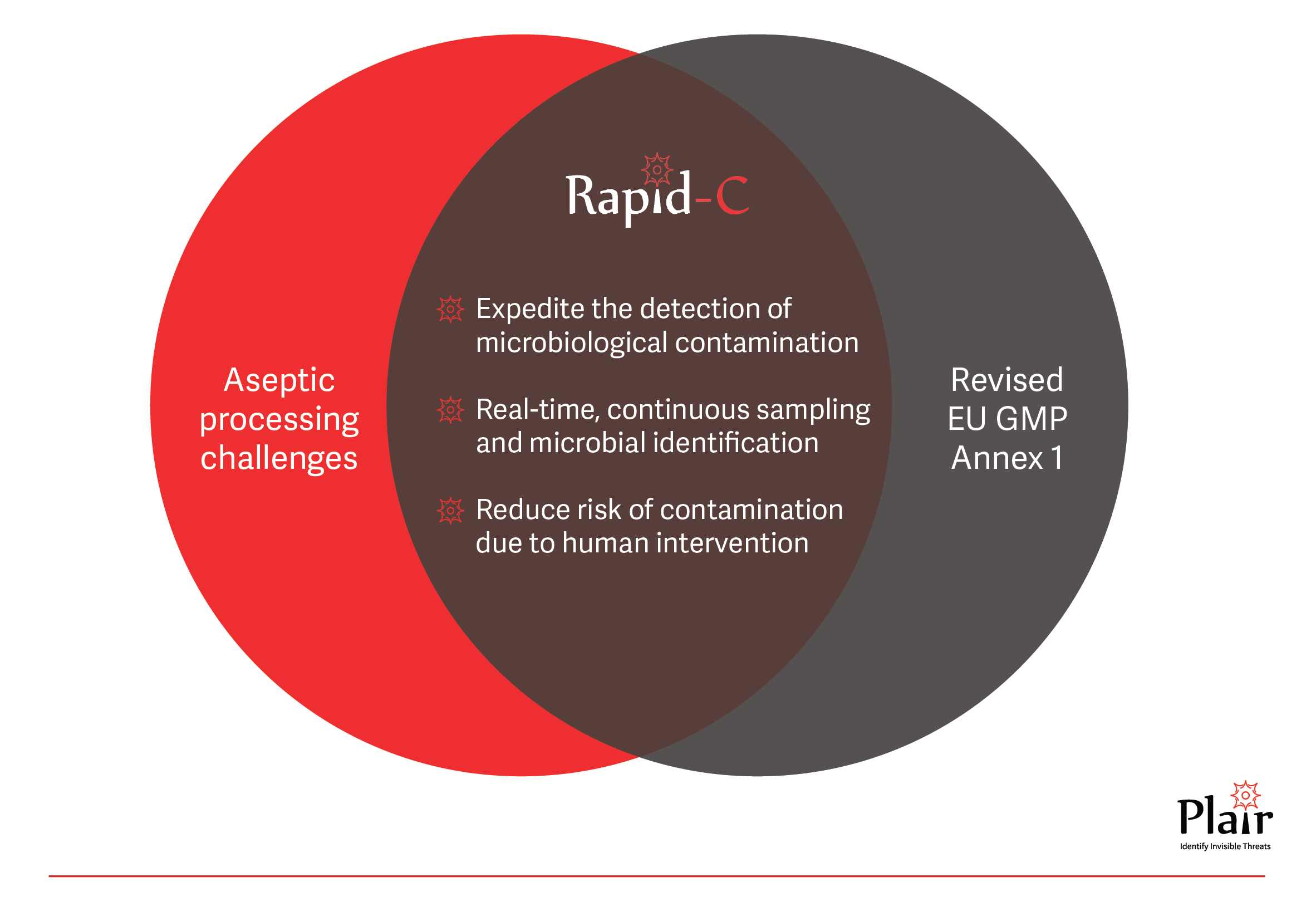
Manufacturers are encouraged to adopt technologies like Restricted Access Barriers Systems (RABS), isolators, robotic systems, rapid/alternative methods, and continuous monitoring systems to fortify product protection against potential contaminants (§2.1). The adoption of alternative monitoring systems is crucial for the swift detection of microbiological issues, reducing risks to the product (§9.28). For products with short shelf life (§10.10), where environmental data may not be readily available, the use of rapid/alternative microbiological methods is recommended. For environmental monitoring, as part of the CCS, it should ensure continuous sampling (§9.24) and microbial identification (§9.31).
Plair’s Rapid-C, is an advanced bio-fluorescent particle counter for real-time microbial detection that fully meets the requirements of the revised Annex 1. It provides real-time viable and total particle counts and enables continuous sampling on standard agar media for microbial identification. This innovative solution offers immediate alerts upon detection of viable particles, enabling proactive interventions. In contrast to compendial methods, Rapid-C allows to save invaluable production days, to accelerate release times, and to improve processes. The Rapid-C‘s holistic approach provides a more comprehensive understanding of the microbial landscape and provides accurate identification data which helps create an effective CCS.
Rapid-C finds indispensable applications in the production of vaccines, life-saving therapies, and various pharmaceutical products manufactured under stringent sterile conditions, including Isolators and RABS. Contamination during production can lead to production halts, extensive financial losses, potential regulatory repercussions, and damage to brand reputation. Rapid-C ensures real-time monitoring, proactive detection, and swift action to mitigate these risks.
In conclusion, Rapid-C emerges as a transformative solution, aligning with the objectives outlined in the revised Annex 1 guidelines, and positioning pharmaceutical manufacturers at the forefront of cutting-edge contamination control strategies. Rapid-C can also be used in other industries such as food and beverage, cosmetics, and aerospace to support their CCS.
For more information and to book your trial instrument, don’t hesitate to contact us at info[AT]plair.ch!